February 2 RxAdvocate February, 2024 — Newsletter
February Stories:
- Top 10 Drug Launches 2024
- NC Discontinuing Anti-Obesity Drug Coverage
- First Ever Frostbite Treatment Approval
- Community Involvement
Fierce Pharma recently released an article on the top 10 new drugs anticipated for 2024. The top spot is held by a product for schizophrenia expected to gain approval in September of this year. KarXT has shown favorable results on improving disease severity in the most recent phase 3 studies with less side effects than current medications approved for this indication.
While schizophrenia is considered a rare disease with 1.2% of the US population impacted, there have been minimal recent drug developments and KarXT could provide the innovation needed in this space. In addition, the drug manufacturer has already begun additional clinical studies to get this product approved for additional indications to expand its footprint into other psychosis treatments. The remainder of the predicted top 10 include products for Alzheimer’s, nonalcoholic steatohepatitis, pulmonary arterial hypertension, lung and breast cancer, cardiomyopathy, RSV vaccination, invasive bladder cancer, chronic COPD and myelodysplastic syndromes.
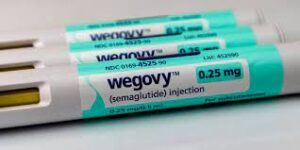
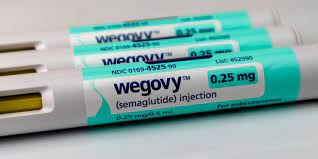
On January 25, 2024, the North Carolina State Health Plan voted in favor of terminating all coverage for glucagon-like peptide-1 (GLP-1) medications when prescribed for weight loss for its roughly 480,000 state employees. This termination of coverage is effective as of April 1, 2024.
The board cited rising costs and a lack of agreement on drug pricing as the reasons for this decision. The NC State Health Plan saw a significant increase in prescriptions for GLP-1 drugs for weight loss in 2023, which led to more than $100 million in spend on these drugs–about 10% of the plan’s payments for all prescriptions. Many state health plans are searching for long term solutions and alternative payment models when considering coverage of anti-obesity medications.
FDA Approves First Ever Treatment for Frostbite

As of February 14th, 2024, the FDA approved the first ever treatment for severe frostbite cases. The medication is Aurlumyn, which is an injection to treat severe frostbite in adults to prevent the risk of finger or toe amputations.
According to the Director of the Division of Cardiology and Nephrology in the FDA’s Center for Drug and Evaluation and Research, “Having this new option provides physicians with a tool that will help prevent the lifechanging amputation of one’s frostbitten fingers or toes.”
Frostbite can range from mild cases which do not require medical intervention to severe cases where both the skin and underlying tissue are frozen and the blood flow stops, which can sometimes require amputation. The active ingredient in Aurlumyn, Iloprost, opens up blood vessels and prevents the blood from clotting. Read more about Aurlumyn here:
RxConnection visits the Big Easy!
This February, RxConnection took on vibrant and cultural New Orleans, Louisiana for Summit 2024! We had a wonderful opportunity to tune in to educational and motivational speakers while also getting the chance to reconnect with our fellow teammates. We caught some Mardi Gras beads and ate a few beignets along the way, too! We are leaving New Orleans feeling inspired and ready to “Make it Matter” for our clients.
Contact us
Orlando.Neal@rxconnectionllc.com





